
USE CASE
It is difficult to find appropriate experts because classifying knowledge and queries are often done manually. By locating subject matter experts and key opinion leaders to involve in research studies and clinical trials, DISQOVER improves finding experts based on expertise built-up in study or work.
Key Opinion Leaders (KOLs) in the pharma industry are Healthcare Professionals (HCPs) with extensive expertise and knowledge in therapeutic areas. They advise companies on a wide range of activities, from drug discovery research and pre-clinical stages to clinical trials and approval for market launch.
It is difficult to find appropriate experts because classifying knowledge and queries are often done manually. By locating subject matter experts and key opinion leaders to involve in research studies and clinical trials, DISQOVER improves finding experts based on expertise built-up in study or work. Moreover, the process of identifying suitable experts is often time-consuming. Experts can be identified by manually reading publications and social media posts for keywords or by searching for experts in databases. This process is time-consuming and error-prone.
By overcoming the problems with available data used to locate experts, DISQOVER shortens identification time to find high-quality matching experts in specific domains. DISQOVER automates classifications and analyzes, links, and infers internal and external data to find matching experts via study, projects, patents, experiences, and more. DISQOVER has developed a new approach to find the most relevant experts that allow users to identify experts automatically with few inputs, even though they are anonymous (i.e., without knowing their name, position, or affiliation). DISQOVER uses a combination of text mining, semantic analysis, and semantic search to identify the most relevant experts in any field. As a result, this improves the quality or caliber of experts engaged, which impacts the likelihood of success.
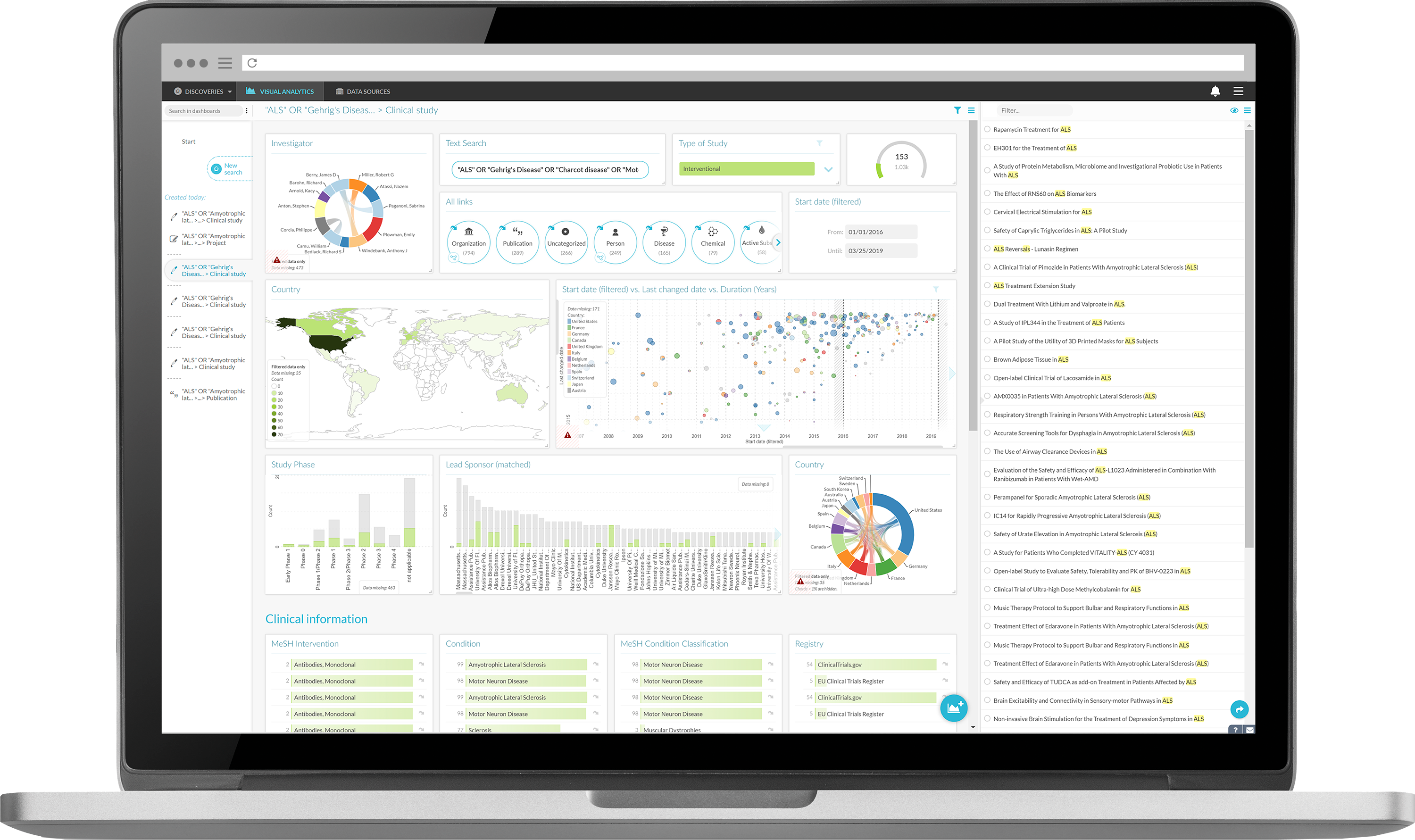
No lost time or frustrations by having to search in different applications.
An improved finding of internal and external experts based on expertise built-up in study or work.
More data to enrich and support an objective expert or expertise selection.
WEBINAR
How to select the right clinical sites that fit your specific clinical study needs? It’s a daunting and time-consuming task.
Experience a live walkthrough of DISQOVER with one of our experts, and get to see how our powerful knowledge discovery platform can help you accelerate your drug development activities.
© 2025 ONTOFORCE All right reserved