
USE CASE
Discover our in-depth resources on how to comply with EMA's IDMP regulations.
Identification of Medicinal Products (IDMP) is one of the most significant regulatory challenges for all pharmaceutical companies operating in Europe. How can companies navigate this journey towards increased patient safety and use it as an opportunity for business transformation?
Learn how to have easier and faster access to the correct data to make your IDMP submission and lay the foundation for building a genuinely insight-driven organization.
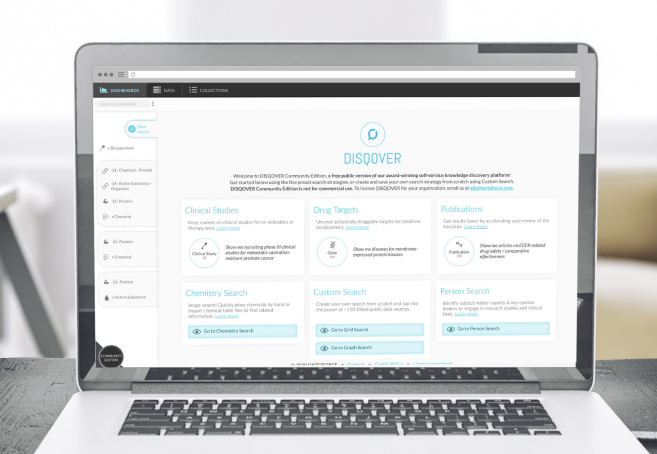
Demo video
Learn how the DISQOVER platform can help you and your team in finding the information they need for IDMP submission.
© 2025 ONTOFORCE All right reserved