
BLOG
With the evolution of technology and data, platforms like DISQOVER are revolutionizing the process of recruiting patients for clinical trials, optimizing strategies and thereby enhancing the efficiency and effectiveness of clinical trials.
Clinical trials play a crucial role in the development of new drugs and therapies. But one of the primary challenges faced by researchers is patient recruitment. Globally, around 80% of trials fail to enroll participants on time and around 55% of trials report termination due to low accrual rate.1
With the evolution of technology and big data, platforms like DISQOVER that leverage the power of data, are revolutionizing the process of recruiting patients for clinical trials, optimizing strategies and thereby enhancing the efficiency and effectiveness of clinical trials. In this article, we’ll dive into the various ways that data and the DISQOVER platform can help in this area.
Trial designers and researchers can turn to data to assist them in the clinical trial design process. Utilizing data, especially historical data, may reveal crucial insights that can help overcome or mitigate recruitment roadblocks.
By having a look at data points from similar studies in the past, such as trial duration, enrollment duration, enrollment per country or site, endpoints and outcomes, among other data points from literature, trial designers can make informed, data-driven decisions regarding site and endpoint selection and clinical protocol. Armed with this data, trial design can be optimized for patient recruitment and retention.
While leveraging data is essential in order to improve patient recruitment strategies to drive trial success, reviewing and analyzing this vast amount of data is an intricate and time-consuming process. The data spans various domains, including scientific research, epidemiology, patient demographics, real-world evidence, regulatory guidelines, and more. Each piece of information must be carefully examined to ensure the relevance, accuracy, and integrity of the data being used.
The complexity of managing disparate data sets and ensuring they align with the specific goals and constraints of the clinical trial can lead to prolonged timelines in the planning phase. Consequently, the process calls for not just in-depth examination but also synthesis and integration of the information to create a cohesive and comprehensive trial design. It's a labor-intensive endeavor that underscores the critical role data plays in the foundation of any clinical trial and emphasizes the need for advanced systems that can streamline this vital aspect of clinical research.
ONTOFORCE’s powerful knowledge discovery platform, DISQOVER, is such a system. DISQOVER enables users to efficiently integrate data to drive effective clinical trial design and improved patient recruitment strategies. DISQOVER is currently utilized by researchers and trial designers in pharmaceutical organizations across the globe to streamline data management and exploration, enabling them to tackle hurdles related to utilizing data for optimized recruitment.
DISQOVER addresses some of the many challenges related to data and patient recruitment strategies by providing unique features and advantages:
DISQOVER integrates and links data from diverse sources, including clinical trial registries, research publications, clinical trial management systems, internal documents, and more. This integration enables researchers to access a wide range of information and gain comprehensive insights into relevant clinical trial information to inform recruitment strategies that drive success.
DISQOVER utilizes semantic search and linked data technologies to enhance the search experience for data and information. It allows researchers and designers to easily find similar trials, discover new endpoints, and assess eligibility criteria suitability all within one platform. No need for multiple screens or applications.
With DISQOVER’s advanced filtering capabilities, users can quickly narrow down the information that is explicitly relevant to the study they are designing.
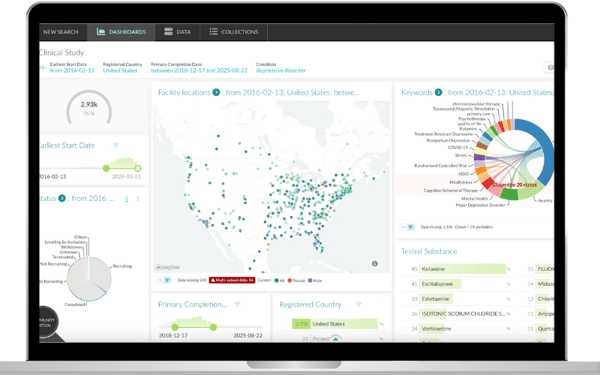
DISQOVER's intuitive and customizable dashboards enable users to visualize and analyze data from various sources easily. This feature supports and accelerates the processes of identifying suitable trial sites, analyzing recruitment strategies, and making data-driven decisions to optimize trial design.
Endpoint selection is a critical step in the design of clinical trials, as it helps in determining the effectiveness of a new treatment or intervention. The endpoints are the specific outcomes or measurements that researchers evaluate to ascertain whether the treatment is having the desired effect.
Streamlining and simplifying eligibility criteria is an important avenue to consider as it can greatly improve the overall efficiency of a study. However, it should always be done with caution to maintain scientific rigor, participant safety, and the study's objectives.
DISQOVER's semantic search capabilities enable researchers to delve into existing literature to identify previously used and validated endpoints for similar studies. This not only informs endpoint selection but also ensures alignment with regulatory guidelines.
In addition to this, thanks to its linked data technology, DISQOVER users can easily understand the underlying connections between different variables, which also helps in selecting the most relevant and responsive endpoints.
Choosing the right site for a clinical trial is crucial as it directly impacts participant recruitment and retention, data quality, adherence to protocol, regulatory compliance, and overall study efficiency.
DISQOVER enables researchers and designers to identify the most suitable sites for their trials based on site data related to previous trial experiences, capabilities, and patient recruitment potential. This ensures optimal site selection and increases the likelihood of successful recruitment and high retention rates.
Additionally, DISQOVER also enables researchers to review historical performance data of potential sites, including recruitment rates, protocol adherence, and outcomes. This helps in identifying the sites with proven track records of success. Further, by accessing data related to quality metrics in DISQOVER, researchers can assess the potential sites' capabilities and infrastructural readiness to conduct the trial, ensuring alignment with the study's requirements.
Patient recruitment in clinical trials poses various challenges, but there are solutions to explore in order to overcome them. DISQOVER's integration of diverse data sources, semantic search capabilities, and data visualization features provide valuable support in dealing with some of the major patient recruitment challenges. Additionally, the ability to gain advanced insights into site and end point selection provides multiple advantages for driving successful trials.
The DISQOVER platform is more than just a technological tool; it's a strategic ally in the complex process of patient recruitment transforming the way researchers approach this vital aspect of clinical research.
1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7342339/
© 2026 ONTOFORCE All right reserved