 CORPORATE
CORPORATE
Connect. Explore. Act.
Accelerate R&D, clinical development, and drug discovery with DISQOVER, the life sciences-native platform that integrates and enriches your data using powerful semantic technologies
We're excited to announce an expanded, multi-year collaboration with Merck KGaA to accelerate data-driven drug discovery. Learn more about the extended partnership >>>
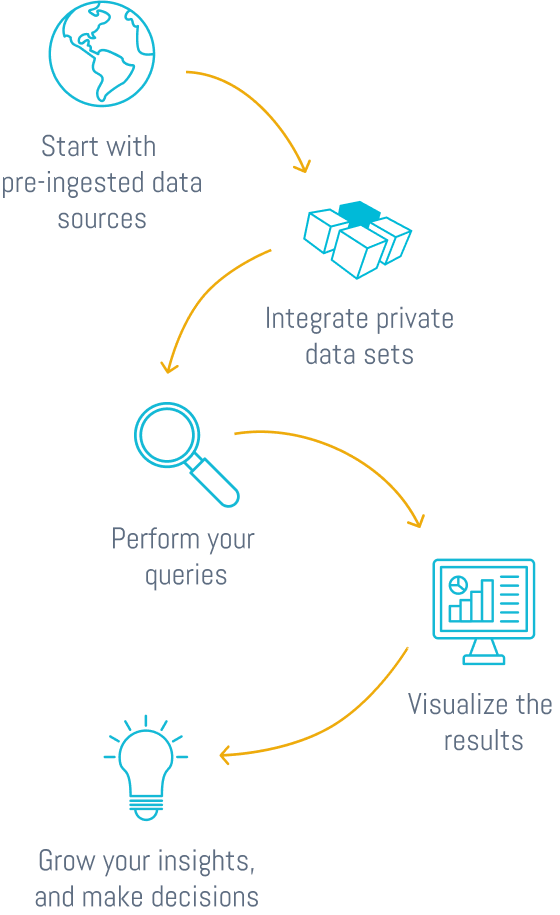
Harmonize, enrich and explore complex biological and business data, all in one single, easy-to-use platform
Growing data volumes across siloed systems create costly delays, missed insights, and bottlenecked innovation. DISQOVER changes that and empowers your organization to move forward with speed and confidence:
Connect multimodal data and more from disparate sources to reveal hidden relationships and insights thanks to ONTOFORCE's powerful knowledge graph technology
Find what matters faster with advanced, semantic search that captures context and meaning, not just keywords. Visualizations and dashboards accelerate interpretation and decision-making.
Your analyses, based upon your own data enriched with the latest available information from over 80 industry sources
Power your decision-making including machine learning models with structured, connected data that reveals patterns and relationships across your entire data ecosystem.
Protect your sensitive data with enterprise-grade security built into every layer of the platform
Process and analyze data in real-time for immediate actionable insights that keep your research moving forward

DISQOVER builds your semantic layer by using the powerful concept of an ontology-based knowledge graph that integrates, link, and structure data.
Combined with AI-based semantic search and natural language processing, siloed data sources are turned into a single, uniform, and high-quality knowledge base.
Thanks to a powerful and easy-to-use interface, scientific researchers become true data citizens.
DISQOVER integrates seamlessly with established core technologies and market-dominant vendors in the bio-sciences industry.
Rapidly identify targets with harmonized multi-omics data. Validate existing targets with relevant public studies and accelerate your discovery pipeline.
Identify and validate biomarkers by connecting genomic, proteomic, and clinical data across integrated data sources.
Gain access to a wealth of data from disparate sources that can be easily compared and visualized insightfully to accelerate and de-risk clinical development.
Streamline inventory analysis, uncover insights to optimize utilization, and enable data-backed decision-making.
Faster, evidence-rich target safety triage and actionable outputs for decision making.
Accelerate drug discovery, clinical trials, manufacturing and regulatory compliance
Optimize contract research and improve data management
Drive crop science, chemicals, and agricultural innovation
Enhance product development and safety testing
Make veterinary research and product development more efficient
Detect new sustainable biotechnology applications faster and easier
DISQOVER has helped us connect data sources, link them, and represent them in a visually appealing way. Moreover, ONTOFORCE takes away the headache of having to manage multiple data sources. With DISQOVER, everything is in one system, and ONTOFORCE takes care of it.
IT Lead at UCB
ONTOFORCE is not giving you just a framework - they give you a massive package of pre-linked public data that you can immediately plug your internal data into. On top of this, DISQOVER’s intuitive interface makes onboarding users straightforward so they can quickly start exploring the linked data.
.png?width=300&height=300&name=Natalie%20Romanov%20PhD%20Merck%20Group%20-%20ONTOFORCE%20(1).png)
Natalie Romanov
Data Strategy Management at Merck Healthcare
Using DISQOVER has certainly been a key way for us to save an awful lot of effort when visualizing data and enabling users to explore the data in a well-received, easy-to-use manner.
Solution Data Architect
Leading pharma company
With ONTOFORCE’s next level search technology, we will be able to safely open up more clinical data for researchers than ever before, helping us to improve our care for children with cancer and to accelerate our pediatric oncology research. We’ve already received great support from ONTOFORCE’s data science experts in implementing DISQOVER so it is tailored to the needs of our organization.
Michiel Kooper
Managing Director IDT (Information Technology, Data Provision, and [Biomedical] Technology) at the Princess Máxima Center for Pediatric Oncology
The Target Assessment Data Lake powered by DISQOVER, represents a transformative step in our mission to bring innovative therapies to patients more efficiently. This initiative has streamlined one of the most critical aspects of our drug discovery process: target evaluation.

Marcin von Grotthuss
Director, Data Integration & Analytics at Takeda Development Center Americas, Inc.
With the DISQOVER user interface, the time it takes to conduct cohort selection went down from weeks to minutes.

Ben Gardner
R&D Lead Data Mesh and Semantic Infrastructure at AstraZeneca
Explore the emerging trends in generative AI within the life sciences industry, the goals companies are striving to achieve with this evolving technology, and the role of knowledge graphs in enabling these advancements.




.png)





© 2025 ONTOFORCE All right reserved